Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 14 junho 2024

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Hepatitis B Foundation
Hepatitis B drug developers chart slow progress, just like in hep C

Hepatitis B drug developers chart slow progress, just like in hep C

LinkedIn Landon Loving 페이지: Biotech pipeline hosts 163
LA Weekend: Haunted Hayride; Jack-O'-Lantern Walk; Pull-A-Plane

Antios rocked as hepatitis B safety signal sparks clinical hold

Landon Loving on LinkedIn: Selecta, Sobi rout gout in pair of

Hepatitis B Foundation

Annalee Armstrong - Journalist Profile - Intelligent Relations

Annalee Armstrong - Journalist Profile - Intelligent Relations

Former J&J R&D chief Mathai Mammen lands at FogPharma
Recomendado para você
-
 some doors gifs : r/RobloxDoors14 junho 2024
some doors gifs : r/RobloxDoors14 junho 2024 -
 Halt Appears at Door 8.. - Roblox Doors14 junho 2024
Halt Appears at Door 8.. - Roblox Doors14 junho 2024 -
 Doors Halt Blank Template - Imgflip14 junho 2024
Doors Halt Blank Template - Imgflip14 junho 2024 -
 Paint Spray Booths: Construction, Types, Applications, and Benefits14 junho 2024
Paint Spray Booths: Construction, Types, Applications, and Benefits14 junho 2024 -
 Frozen: A Song of Doors and Windows – This Builds Character14 junho 2024
Frozen: A Song of Doors and Windows – This Builds Character14 junho 2024 -
 The Office Is Dead. Get ready for the commercial real…, by Courtney Rubin14 junho 2024
The Office Is Dead. Get ready for the commercial real…, by Courtney Rubin14 junho 2024 -
 Here Come the Zoomers: Silicon Valley Greets a New Generation of Teen Founders — The Information14 junho 2024
Here Come the Zoomers: Silicon Valley Greets a New Generation of Teen Founders — The Information14 junho 2024 -
 kftI9GR.gif14 junho 2024
kftI9GR.gif14 junho 2024 -
 ETH Prof Lehnerer Haus Halt — All Projects14 junho 2024
ETH Prof Lehnerer Haus Halt — All Projects14 junho 2024 -
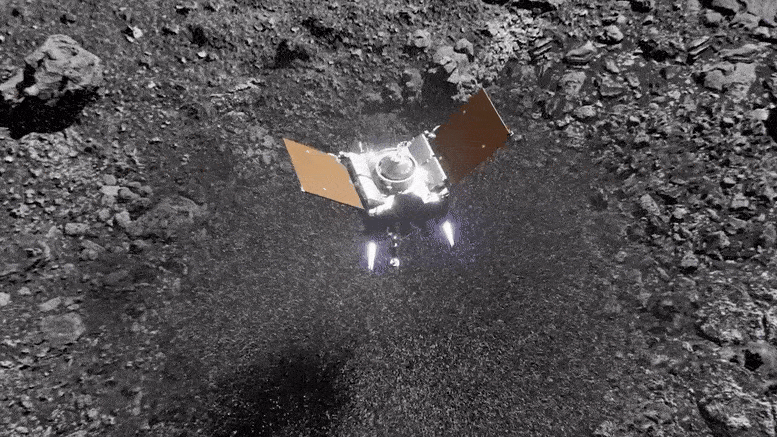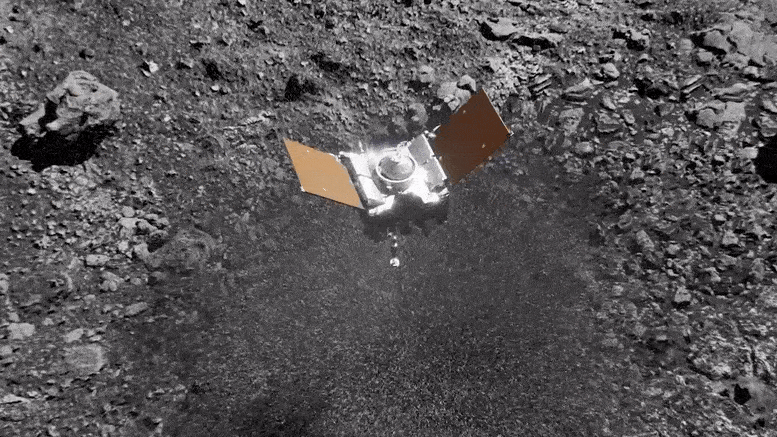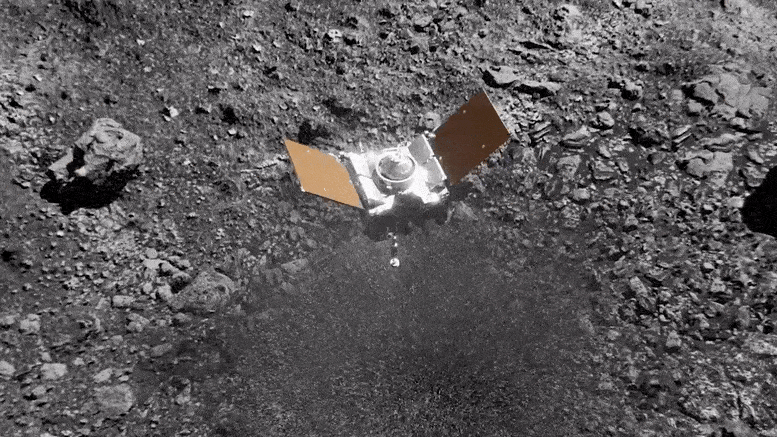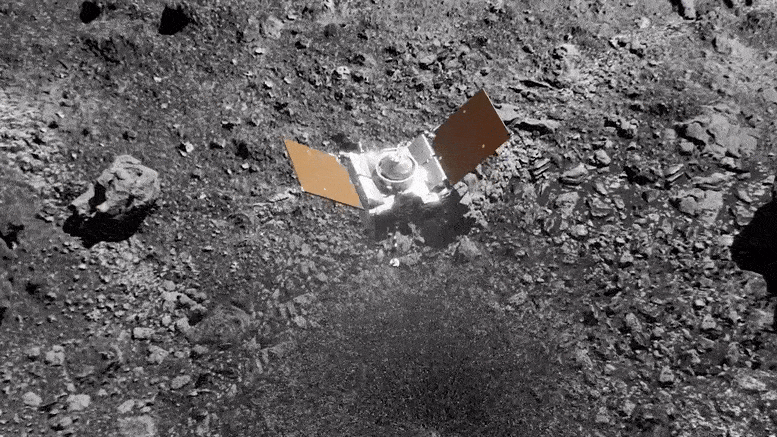 Preparing for Asteroid Bennu: NASA's OSIRIS-REx Astromaterials Lab Opens Doors to Media14 junho 2024
Preparing for Asteroid Bennu: NASA's OSIRIS-REx Astromaterials Lab Opens Doors to Media14 junho 2024
você pode gostar
-
 Akuyaku Reijou nanode Last Boss wo Kattemimashita14 junho 2024
Akuyaku Reijou nanode Last Boss wo Kattemimashita14 junho 2024 -
Roblox Stock Price Spikes 16% in Public Trading Debut14 junho 2024
-
Oreshura (Ore no Kanojo to Osananajimi ga Shuraba Sugiru)14 junho 2024
-
 Great Opening Lines14 junho 2024
Great Opening Lines14 junho 2024 -
 Faculdade Sogipa Oficial14 junho 2024
Faculdade Sogipa Oficial14 junho 2024 -
 Roblox chegou às plataformas PlayStation 5 - Record Gaming14 junho 2024
Roblox chegou às plataformas PlayStation 5 - Record Gaming14 junho 2024 -
 How to Install Skyrim Mods (with Pictures) - wikiHow14 junho 2024
How to Install Skyrim Mods (with Pictures) - wikiHow14 junho 2024 -
 Fogo Ilustração PNG , Fogo, Desenho Animado, Ilustração Imagem PNG14 junho 2024
Fogo Ilustração PNG , Fogo, Desenho Animado, Ilustração Imagem PNG14 junho 2024 -
 Lies of P All Special Weapon Showcase + Moveset14 junho 2024
Lies of P All Special Weapon Showcase + Moveset14 junho 2024 -
 Patinete Masculino 3 Rodas Com Cesto Som E Led Havan Toys - HBR044514 junho 2024
Patinete Masculino 3 Rodas Com Cesto Som E Led Havan Toys - HBR044514 junho 2024
