IJMS, Free Full-Text
Por um escritor misterioso
Last updated 06 novembro 2024
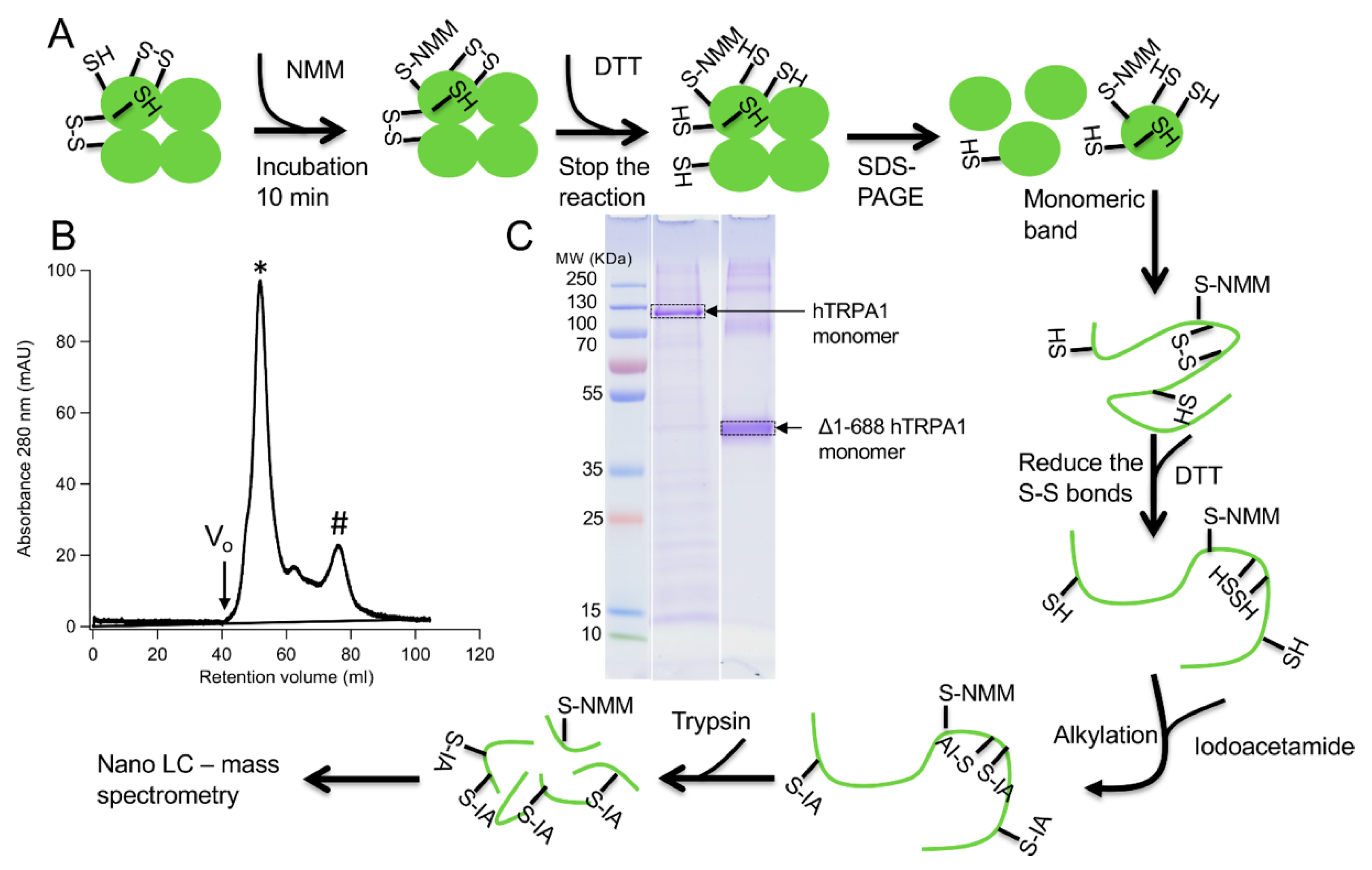
The human Transient Receptor Potential A1 (hTRPA1) ion channel, also known as the wasabi receptor, acts as a biosensor of various potentially harmful stimuli. It is activated by a wide range of chemicals, including the electrophilic compound N-methylmaleimide (NMM), but the mechanism of activation is not fully understood. Here, we used mass spectrometry to map and quantify the covalent labeling in hTRPA1 at three different concentrations of NMM. A functional truncated version of hTRPA1 (Δ1-688 hTRPA1), lacking the large N-terminal ankyrin repeat domain (ARD), was also assessed in the same way. In the full length hTRPA1, the labeling of different cysteines ranged from nil up to 95% already at the lowest concentration of NMM, suggesting large differences in reactivity of the thiols. Most important, the labeling of some cysteine residues increased while others decreased with the concentration of NMM, both in the full length and the truncated protein. These findings indicate a conformational switch of the proteins, possibly associated with activation or desensitization of the ion channel. In addition, several lysines in the transmembrane domain and the proximal N-terminal region were labeled by NMM, raising the possibility that lysines are also key targets for electrophilic activation of hTRPA1.
IJMS, Free Full-Text
IJMS, Free Full-Text
IJMS, Free Full-Text
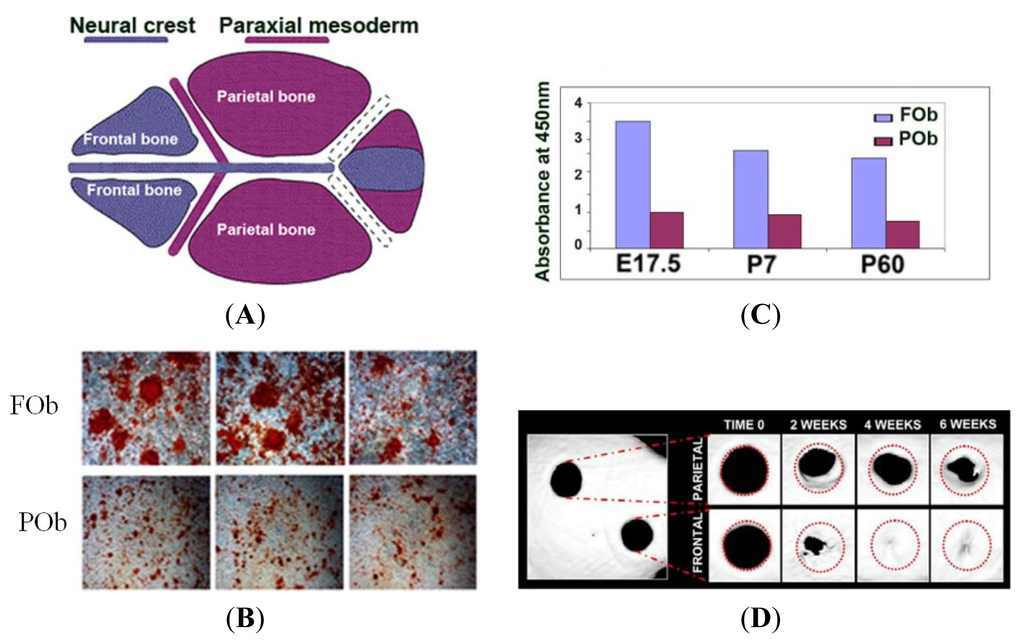
IJMS, Free Full-Text
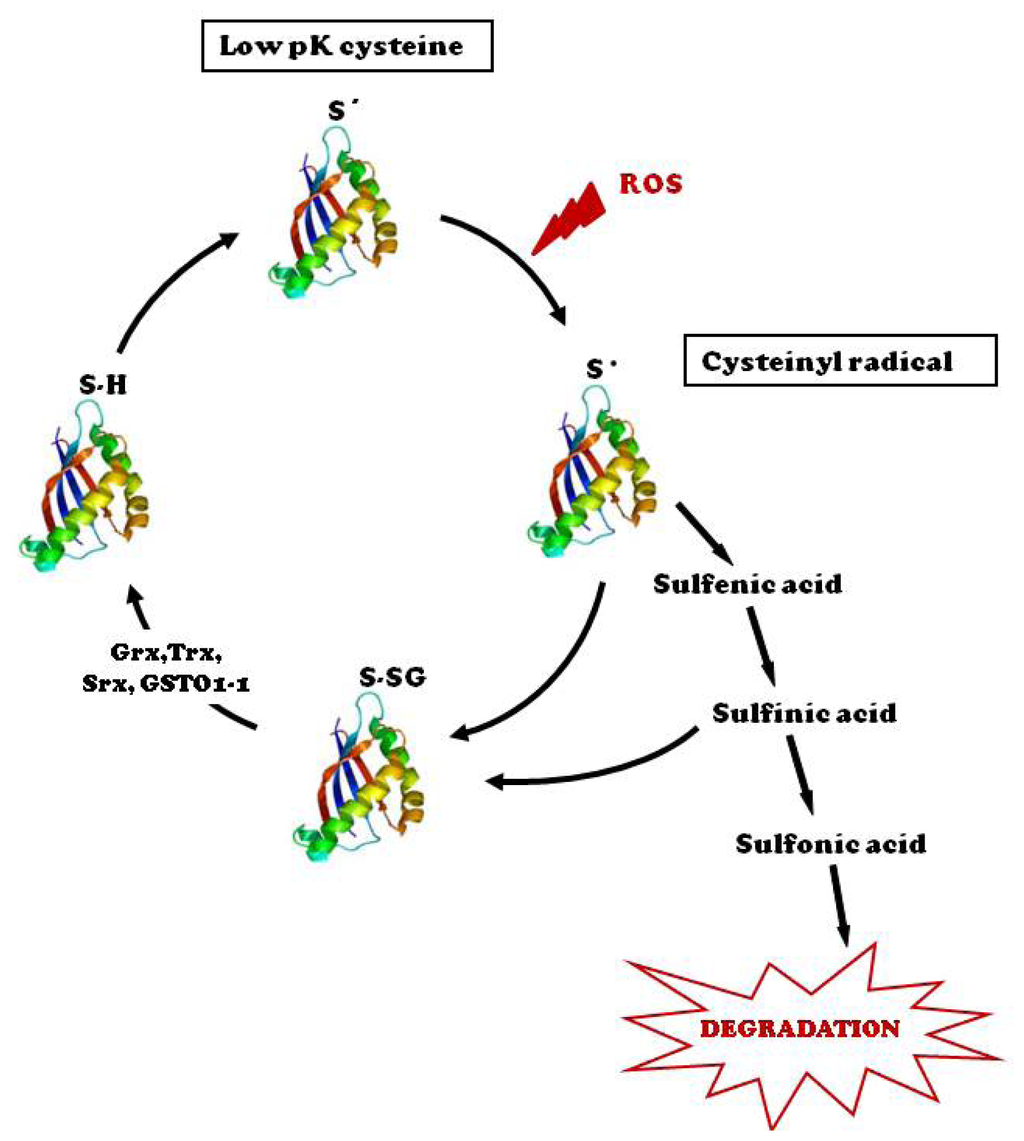
IJMS, Free Full-Text
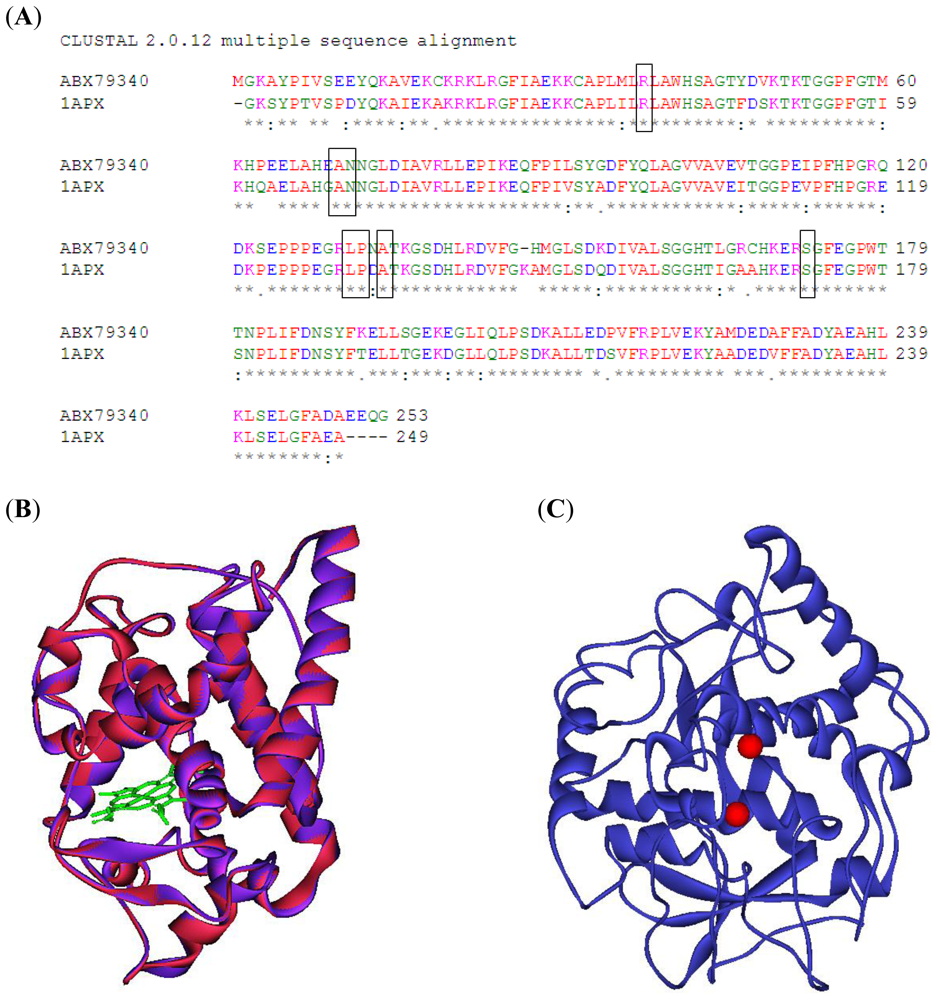
IJMS, Free Full-Text
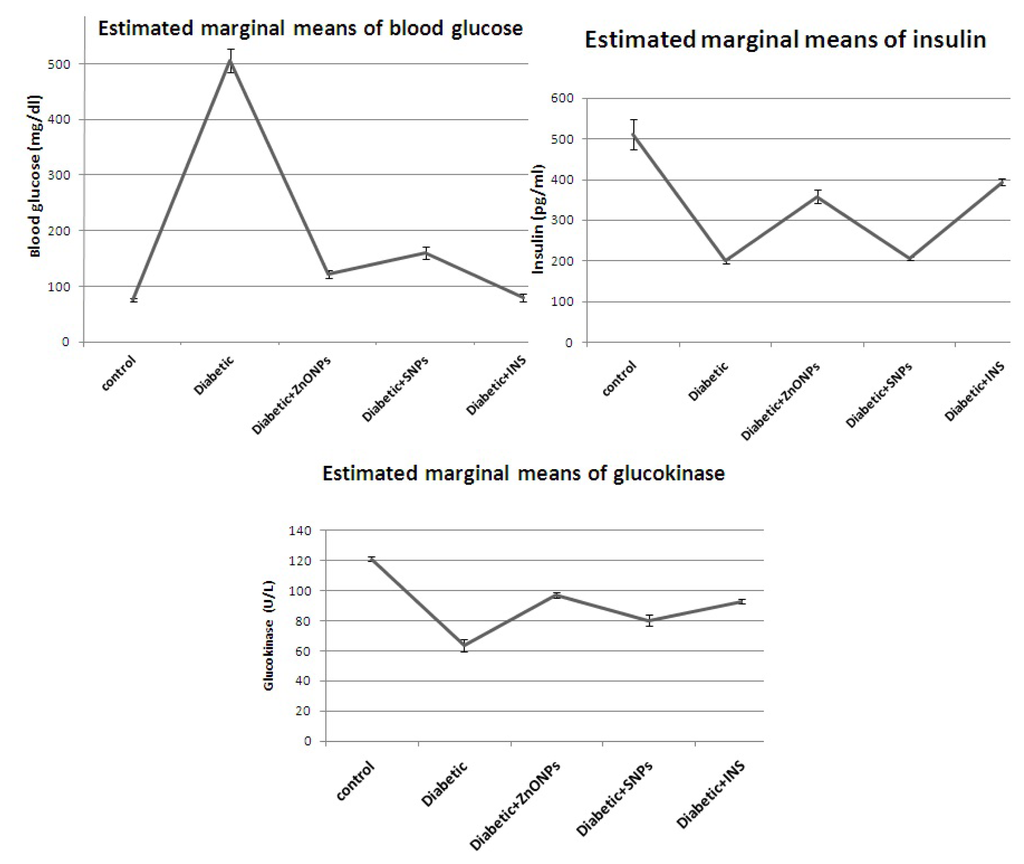
IJMS, Free Full-Text
pka creb signaling pathway - Clip Art Library
IJMS, Free Full-Text
Mpep Substantially Free - Colaboratory
Recomendado para você
-
 logotipo nm. letra nm. design de logotipo de letra nmm. iniciais nmm logotipo ligado com círculo e logotipo monograma maiúsculo. tipografia nmm para marca de tecnologia, negócios e imóveis. 9025548 Vetor no06 novembro 2024
logotipo nm. letra nm. design de logotipo de letra nmm. iniciais nmm logotipo ligado com círculo e logotipo monograma maiúsculo. tipografia nmm para marca de tecnologia, negócios e imóveis. 9025548 Vetor no06 novembro 2024 -
 Nmm: Over 10 Royalty-Free Licensable Stock Illustrations & Drawings06 novembro 2024
Nmm: Over 10 Royalty-Free Licensable Stock Illustrations & Drawings06 novembro 2024 -
 Chris Shipping 🚢🚢 on X: $NM AFs take private acquisition of NM closed today. Great news for $NMM as the $NM overhang is gone and now AFs equity stake in $NMM is06 novembro 2024
Chris Shipping 🚢🚢 on X: $NM AFs take private acquisition of NM closed today. Great news for $NMM as the $NM overhang is gone and now AFs equity stake in $NMM is06 novembro 2024 -
 What does NMM mean? - NMM Definitions06 novembro 2024
What does NMM mean? - NMM Definitions06 novembro 2024 -
 Space Group 129: P4/nmm; P 4/n m m06 novembro 2024
Space Group 129: P4/nmm; P 4/n m m06 novembro 2024 -
 How to convert KN/m to N/mm, N/mm to KN/mm06 novembro 2024
How to convert KN/m to N/mm, N/mm to KN/mm06 novembro 2024 -
 Water pump - NMM Series - MAS - DAF - electric / centrifugal / industrial06 novembro 2024
Water pump - NMM Series - MAS - DAF - electric / centrifugal / industrial06 novembro 2024 -
 File:Moonlight Scene - Ships Saluting NMM NMMG BHC1074.jpg - Wikipedia06 novembro 2024
File:Moonlight Scene - Ships Saluting NMM NMMG BHC1074.jpg - Wikipedia06 novembro 2024 -
A) Fluorescence intensity of NMM at 608 nm in the presence of distinct06 novembro 2024
-
 File:HMS Temeraire 1886 NMM NMMG BHC3653.jpg - Wikimedia Commons06 novembro 2024
File:HMS Temeraire 1886 NMM NMMG BHC3653.jpg - Wikimedia Commons06 novembro 2024
você pode gostar
-
 Pin by Renato Alves on Digimon (Pt. 01) in 202306 novembro 2024
Pin by Renato Alves on Digimon (Pt. 01) in 202306 novembro 2024 -
 Sugoi LITE on X: TV Anime Classroom of the Elite III (Season 306 novembro 2024
Sugoi LITE on X: TV Anime Classroom of the Elite III (Season 306 novembro 2024 -
/cdn.vox-cdn.com/photo_images/6691744/20120428_ajl_aw6_044.jpg) 2012 NBA Playoffs: Eastern Conference Preview - Detroit Bad Boys06 novembro 2024
2012 NBA Playoffs: Eastern Conference Preview - Detroit Bad Boys06 novembro 2024 -
 Bebe reborn realista menino +140 anúncios na OLX Brasil06 novembro 2024
Bebe reborn realista menino +140 anúncios na OLX Brasil06 novembro 2024 -
 Can someone make this a logo and send me the link : r/bloxfruits06 novembro 2024
Can someone make this a logo and send me the link : r/bloxfruits06 novembro 2024 -
 Watch Avatar: The Last Airbender Online, Season 3 (2007)06 novembro 2024
Watch Avatar: The Last Airbender Online, Season 3 (2007)06 novembro 2024 -
 The Vampire Diaries Por onde anda o elenco da série?06 novembro 2024
The Vampire Diaries Por onde anda o elenco da série?06 novembro 2024 -
 Volante Logitech G29 + Juego Gran Turismo 7. PLAYSTATION 406 novembro 2024
Volante Logitech G29 + Juego Gran Turismo 7. PLAYSTATION 406 novembro 2024 -
 Subway Surfers 3.11.0 APK Download06 novembro 2024
Subway Surfers 3.11.0 APK Download06 novembro 2024 -
 How to Catch the Loyal Three in The Teal Mask - Pokemon Scarlet and Violet Guide - IGN06 novembro 2024
How to Catch the Loyal Three in The Teal Mask - Pokemon Scarlet and Violet Guide - IGN06 novembro 2024