Life Sciences Commissioning, Qualification and Validation
Por um escritor misterioso
Last updated 08 novembro 2024

Commissioning, Qualification and Validation (CQV) are requirements of modern facilities within the Life Science industry. Be it a Medical Device

Commissioning, Qualification and Validation: A GMP Approach
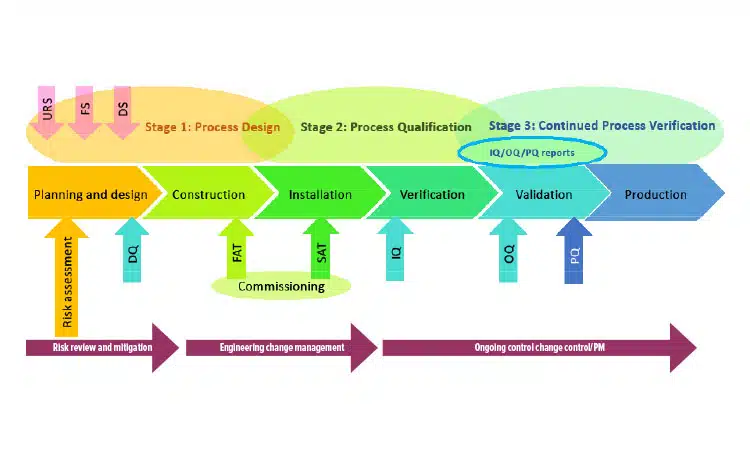
Qualifying a New GMP Facility: From Pitfalls to Best Practices
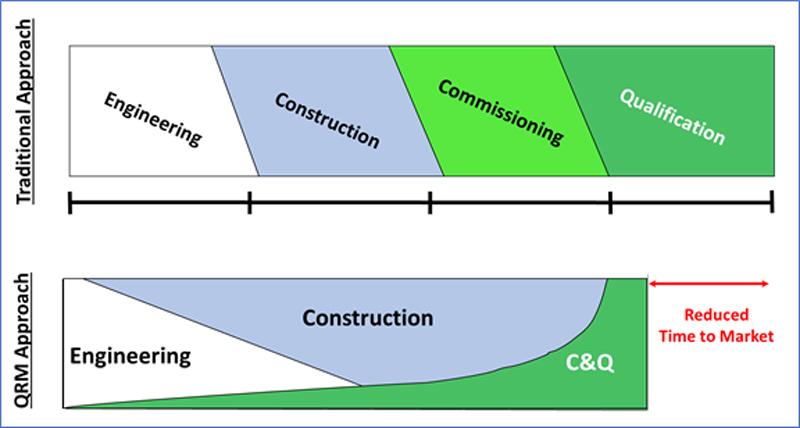
Commissioning & Qualification: Debunking the Myths – No deviation
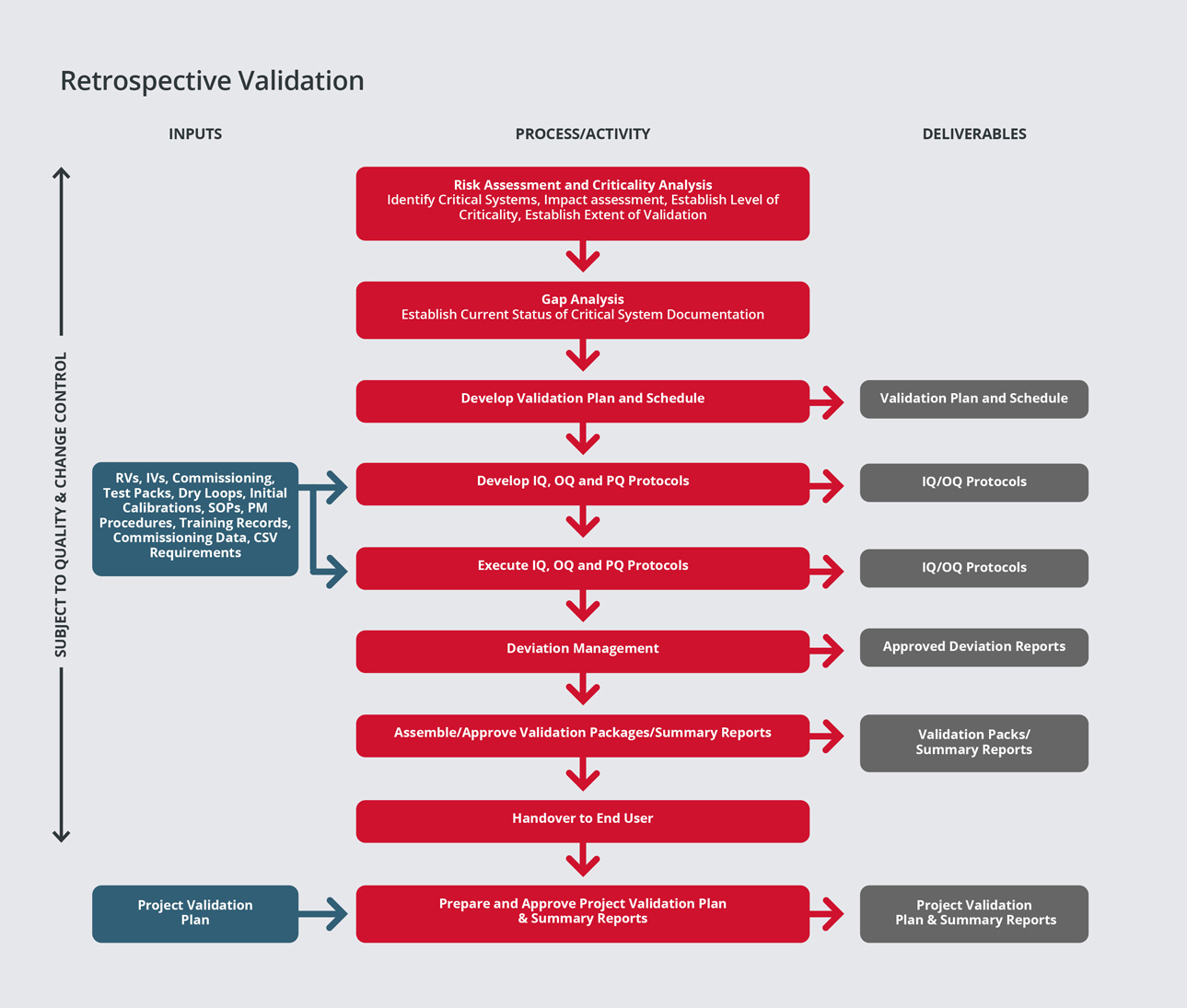
Qualification / Validation - SPGL

Commissioning, Qualification, and Validation

Commissioning, Qualification & Validation

Commissioning, Qualification and Validation: A GMP Approach , Browne, Priscilla

Commissioning, Qualification, and Validation

Commissioning, Qualification and Validation - PharmaLex
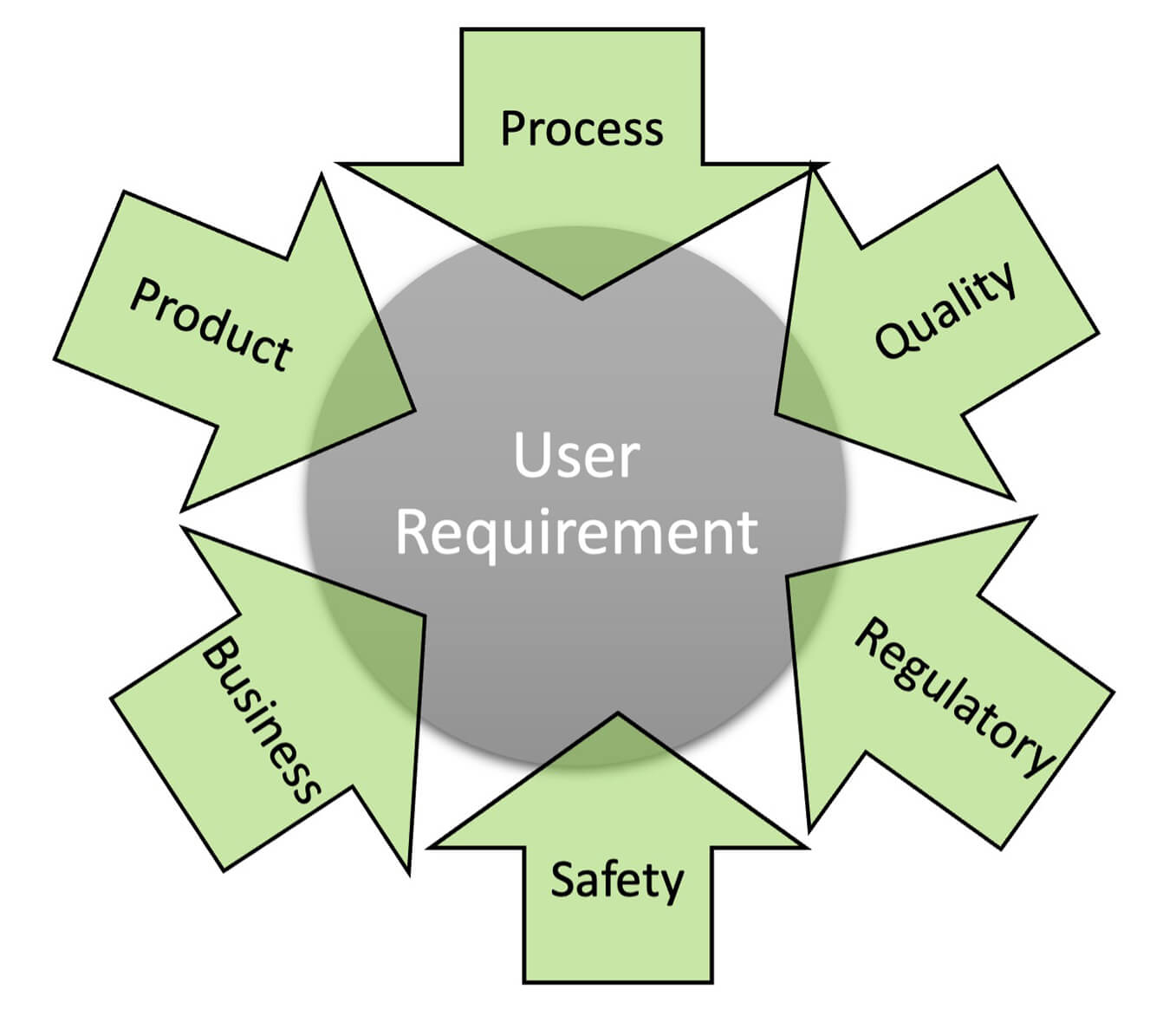
A Successful Q-Model for New mRNA Line Commissioning & Qualification: Pt. 1

Commissioning, Qualification and Validation. - CSV Life Science

Overview of Validation in Pharma_Katalyst HLS
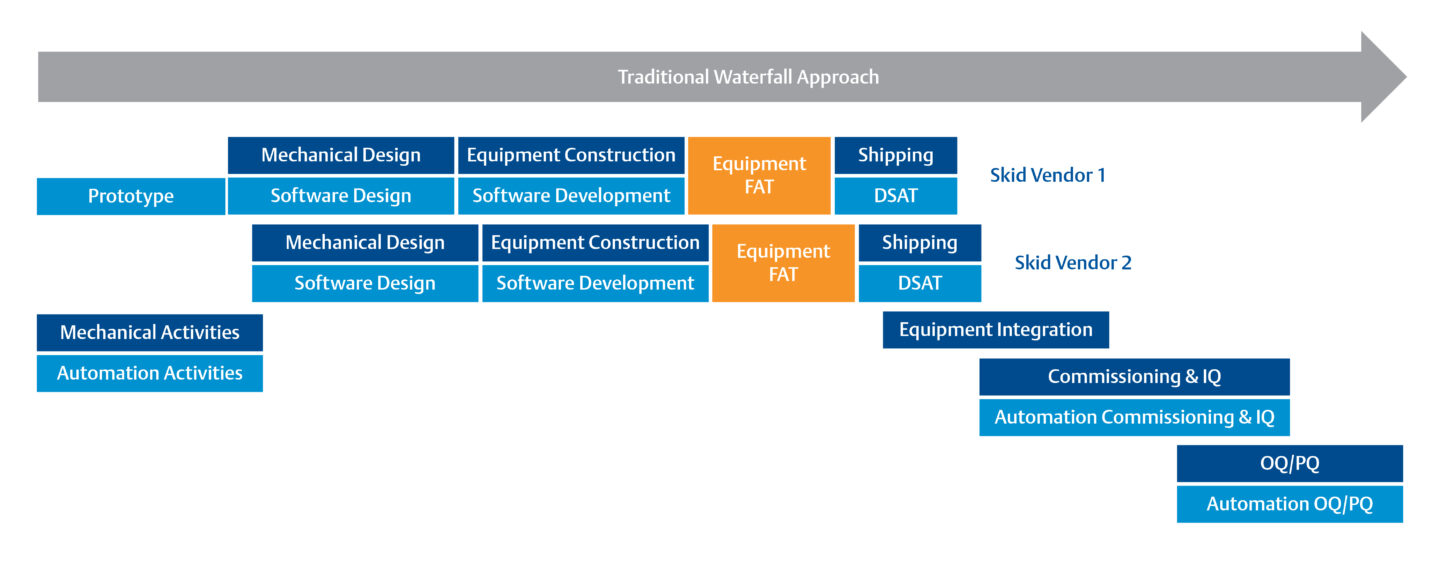
Shorten the Equipment Validation Process to Bring Projects Online Faster
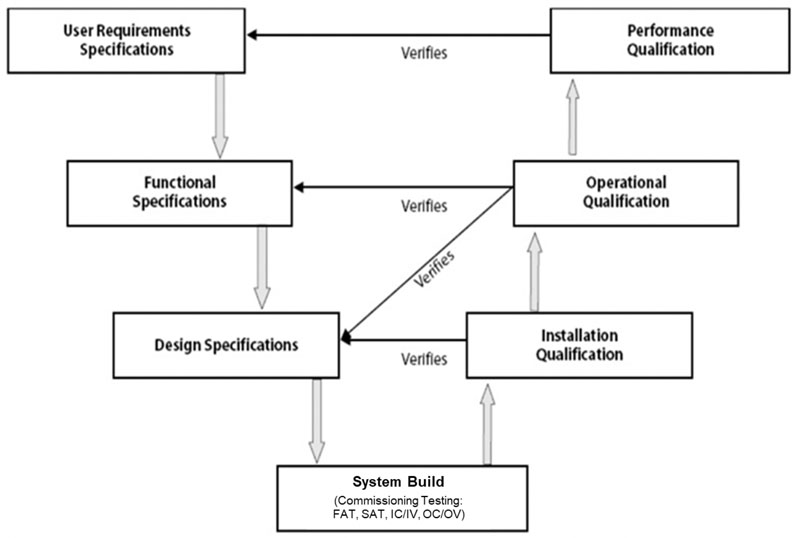
The right validation model: practical considerations and applications
Recomendado para você
-
![OBSOLETE] Select versions of old ICQ clients work! Here's how to](https://wink.messengergeek.com/uploads/default/original/2X/e/e797ad0ab6709218b412d623390fa2ddc9f2ef7f.png) OBSOLETE] Select versions of old ICQ clients work! Here's how to08 novembro 2024
OBSOLETE] Select versions of old ICQ clients work! Here's how to08 novembro 2024 -
 Beginners' Harp & Lyre Christmas Collection: Simple and Beautiful Harmonies for 15 strings tuned to the key of C (Good Old Tunes Harp Music)08 novembro 2024
Beginners' Harp & Lyre Christmas Collection: Simple and Beautiful Harmonies for 15 strings tuned to the key of C (Good Old Tunes Harp Music)08 novembro 2024 -
 Links com abuso infantil ficam no topo de buscas de Google e Bing08 novembro 2024
Links com abuso infantil ficam no topo de buscas de Google e Bing08 novembro 2024 -
 Jungle Safari Wild One Birthday Chip Bag Digital File DIY08 novembro 2024
Jungle Safari Wild One Birthday Chip Bag Digital File DIY08 novembro 2024 -
 ICQ Chat-page08 novembro 2024
ICQ Chat-page08 novembro 2024 -
Также ссылка в шапке профиля❤️ Давайте общаться в ICQ, устанавливай приложение.08 novembro 2024
-
 Gracias Por Su Compra Promo Code Coupon Code Tarjeta De08 novembro 2024
Gracias Por Su Compra Promo Code Coupon Code Tarjeta De08 novembro 2024 -
 Invitation Homes08 novembro 2024
Invitation Homes08 novembro 2024 -
 Depois de polêmicas do WhatsApp, ICQ retorna e volta a ganhar adeptos08 novembro 2024
Depois de polêmicas do WhatsApp, ICQ retorna e volta a ganhar adeptos08 novembro 2024 -
 Princesses Frozen Party Digital Birthday Invitation EDITABLE - Norway08 novembro 2024
Princesses Frozen Party Digital Birthday Invitation EDITABLE - Norway08 novembro 2024
você pode gostar
-
 SAD TROLL FACE Postcard for Sale by Abusive-materia08 novembro 2024
SAD TROLL FACE Postcard for Sale by Abusive-materia08 novembro 2024 -
 Lord e tickles zapped by Bc320903871 on DeviantArt08 novembro 2024
Lord e tickles zapped by Bc320903871 on DeviantArt08 novembro 2024 -
![Zugzwang's Last Move Trap :: Trap - Mousehunt Weapon - Mousehunt Database & Guide Info [DBG]](https://img.dbgames.info/mousehunt/item/zugzwangs_last_move_large.jpg) Zugzwang's Last Move Trap :: Trap - Mousehunt Weapon - Mousehunt Database & Guide Info [DBG]08 novembro 2024
Zugzwang's Last Move Trap :: Trap - Mousehunt Weapon - Mousehunt Database & Guide Info [DBG]08 novembro 2024 -
 Angry Cat Images Free Photos, PNG Stickers, Wallpapers & Backgrounds - rawpixel08 novembro 2024
Angry Cat Images Free Photos, PNG Stickers, Wallpapers & Backgrounds - rawpixel08 novembro 2024 -
 Bubble Hit - Jogue Online no08 novembro 2024
Bubble Hit - Jogue Online no08 novembro 2024 -
 New Attraction (Uncensored Version) by iWTBAT on Newgrounds08 novembro 2024
New Attraction (Uncensored Version) by iWTBAT on Newgrounds08 novembro 2024 -
:max_bytes(150000):strip_icc()/TermDefinitions_IncrementalCapitalOutputRatio_colorv1-7040098af019497bbb073f04f867fc6e.png) Incremental Capital Output Ratio (ICOR): Definition and Formula08 novembro 2024
Incremental Capital Output Ratio (ICOR): Definition and Formula08 novembro 2024 -
 Battle Master trophy in Resident Evil Code: Veronica X HD08 novembro 2024
Battle Master trophy in Resident Evil Code: Veronica X HD08 novembro 2024 -
 Relógio Magnum - Dourado08 novembro 2024
Relógio Magnum - Dourado08 novembro 2024 -
Poki Naya Garden Grove CA08 novembro 2024

